How do we mathematically model random events? We use probabilities and large number of events. For example, by throwing a one hundred dice with a yellow spot on one face of each dice, we can use probabilities and large number of events to produce a graph that the event is completely random with is radioactive decay.
We can deduce an equation for radioactive decay:
Number of decayed atoms
rate of change in number of undecayed atoms.
This equation is N 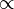 dN/dt
dN/dt
Therefore, we can produce the equation:
dN/dt = –λN
With λ being the decay constant. One problem with our formula is that counting the number of decayed atoms is not easy. From integrating the above equation, we can produce the following equation:
N = N0 x e^-λt
Which can be seen more clearly here:
 |
| Link to webpage |
A = A0 x e^-λt
Basically, you need to swap the ‘N’s with ‘A’s which A is the activity of the source. λ is the decay constant and only depends on the radioactive substance. This means each radioactive substance has it’s own decay constant.
It is important to make sure λ and t both have the same unit. For example, if λ is in /s, t must also be in /s. If λ is in /year, t must also be in /year.
Example
- dN/dt = –λN
- dN/dt = -0.015 x 2.4×10^8
- dN/dt = -3.6×10^6 per second
- N = N0 x e^-λt
- N = 2.4×10^8 x e^-0.015×17.2
- N = 1.9×10^8 atoms
- A = A0 x e^-λt
- A = 3.6×10^6 x e^-0.015×17.2
- A = 2.8×10^6 atoms per second
As a short summary at the moment, we have learnt that:
- Number of atoms left = Number of atoms at t=0 x e^-λt
- Activity = Activity of atoms at t = 0 x e^-λt
- Rate of decay is proportional to –λN (dN/dt = –λN)
- λ is the decay constant of the radioactive material. It is the proportion of atoms decaying per unit time.
Half Life
To Find The Half Life
- N = N0 x e^-λ(t=1/2) where (t=1/2) is the half life.
- N/N0 = e^-λ(t=1/2)
- 1/2 = e^-λ(t=1/2)
- ln(1/2) = –λ x (t=1/2)
- (t=1/2) = ln(1/2) / –λ
Half life= 0.693 / λ
An Example
An isotope of a radioactive element has a value for the decay constant of 0.0917/day. This means that a proportion of the 0.0917 of any sample of this element will decay per day. Calculate the half life for a sample of this isotope.
- (t=1/2) = 0.693/0.0917 = 7.56 days




